
Healthcare know-how firm Aktiia has obtained FDA 510(okay) clearance for its over-the-counter cuffless blood stress monitor, G0 Blood Stress Monitoring System, also referred to as the Hilo Band.
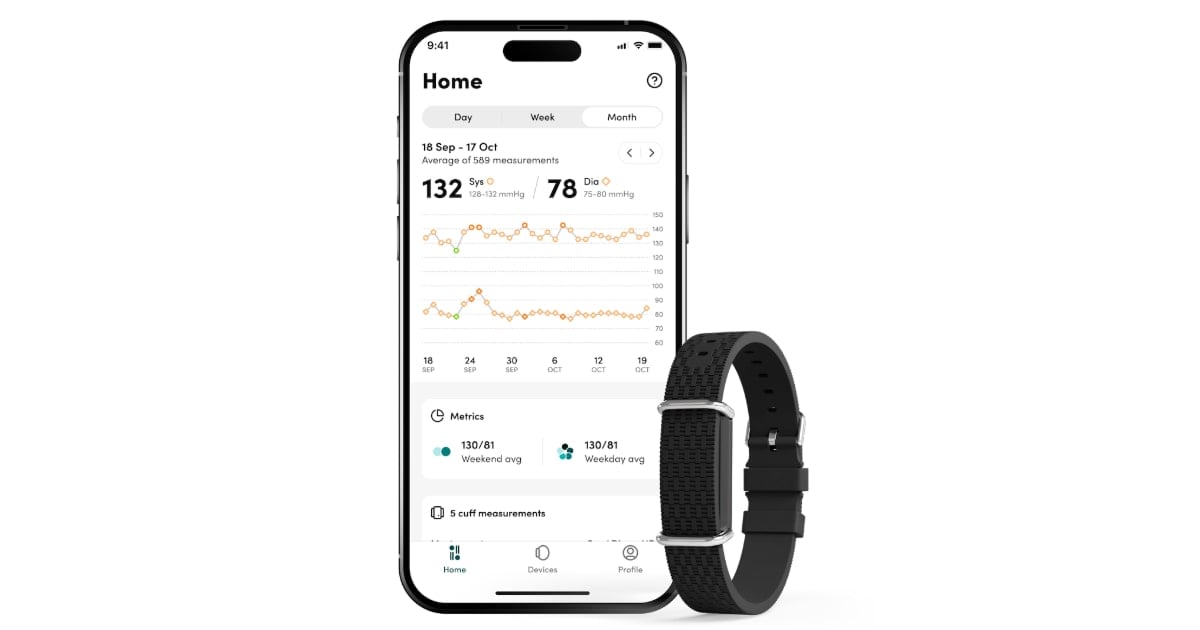
Aktiia’s blood stress system, recognized below the Hilo model in Europe, is a wearable that makes use of an optical sensor to gather information from one’s wrist. That information is then despatched to the Hilo app and Hilo’s cloud server, the place algorithms estimate the wearer’s blood stress utilizing pulse wave evaluation.
The corporate says the evaluation examines the distinctive type of stress exerted by every heartbeat by the wearer’s blood vessels.
The wrist wearable repeatedly collects one’s blood stress all through the day, however does so solely when they’re nonetheless.
The product, which is already CE marked as a Class IIa medical machine for monitoring blood stress in adults in Europe, might be accessible to U.S. shoppers in 2026.
“This milestone is deeply private for us as founders and profoundly necessary for everybody at Aktiia. From day one, our mission has been to deal with the worldwide hypertension disaster by know-how. With this FDA clearance, we’re one step nearer to realizing our imaginative and prescient: a future the place wholesome blood stress is accessible to all,” Dr. Josep Solà, cofounder and chief know-how officer at Aktiia, instructed MobiHealthNews.
“What makes this second actually historic is not only the clearance itself, however the truth that the FDA has authorised the Aktiia machine for over-the-counter use, no prescription required. It is a uncommon and demanding bar to fulfill, and a transparent sign that our know-how shouldn’t be solely clinically validated however prepared for real-world, large-scale deployment.”
Dr. Mattia Bertschi, Aktiia cofounder and CEO, instructed MobiHealthNews that the FDA clearance helps the corporate develop its attain.
“This opens a brand new marketplace for us, however extra importantly, it confirms that Aktiia’s answer is protected, intuitive, and designed for the individuals who want it most. We imagine that is just the start, and that cuffless, wearable, and over-the-counter blood stress monitoring will quickly change into the worldwide normal,” Bertschi stated.
“We’re working day and night time to make sure that Hilo by Aktiia would not simply take part on this transformation … however leads it.”
THE LARGER TREND
In Might, Aktiia scored $42 million in an oversubscribed Collection B funding spherical, bringing its complete increase to greater than $100 million.
Final 12 months, Aktiia accomplished a CHF 27 million ($30 million) funding spherical and obtained CE mark in Europe for its calibration-free know-how CALFREE, which permits blood stress information to be collected utilizing inputs from optical sensors generally present in smartwatches and cameras of economic smartphones.
The Swiss firm stated that approval of its CALFREE tech will allow it to supply integration of its medical-grade optical blood stress monitoring to third-party corporations creating client units, equivalent to smartwatches, smartphone cameras and sensible bands.
In 2021, the corporate raised $17.5 million in Collection A funding, and the 12 months earlier than, it secured $6.1 million (CHF 6 million) in funding.
The corporate, based in 2018, was a spin-out of CSEM, a Swiss analysis and improvement middle.





Leave a Reply